The Circulo Management Platform: A Solution Built for Life Sciences
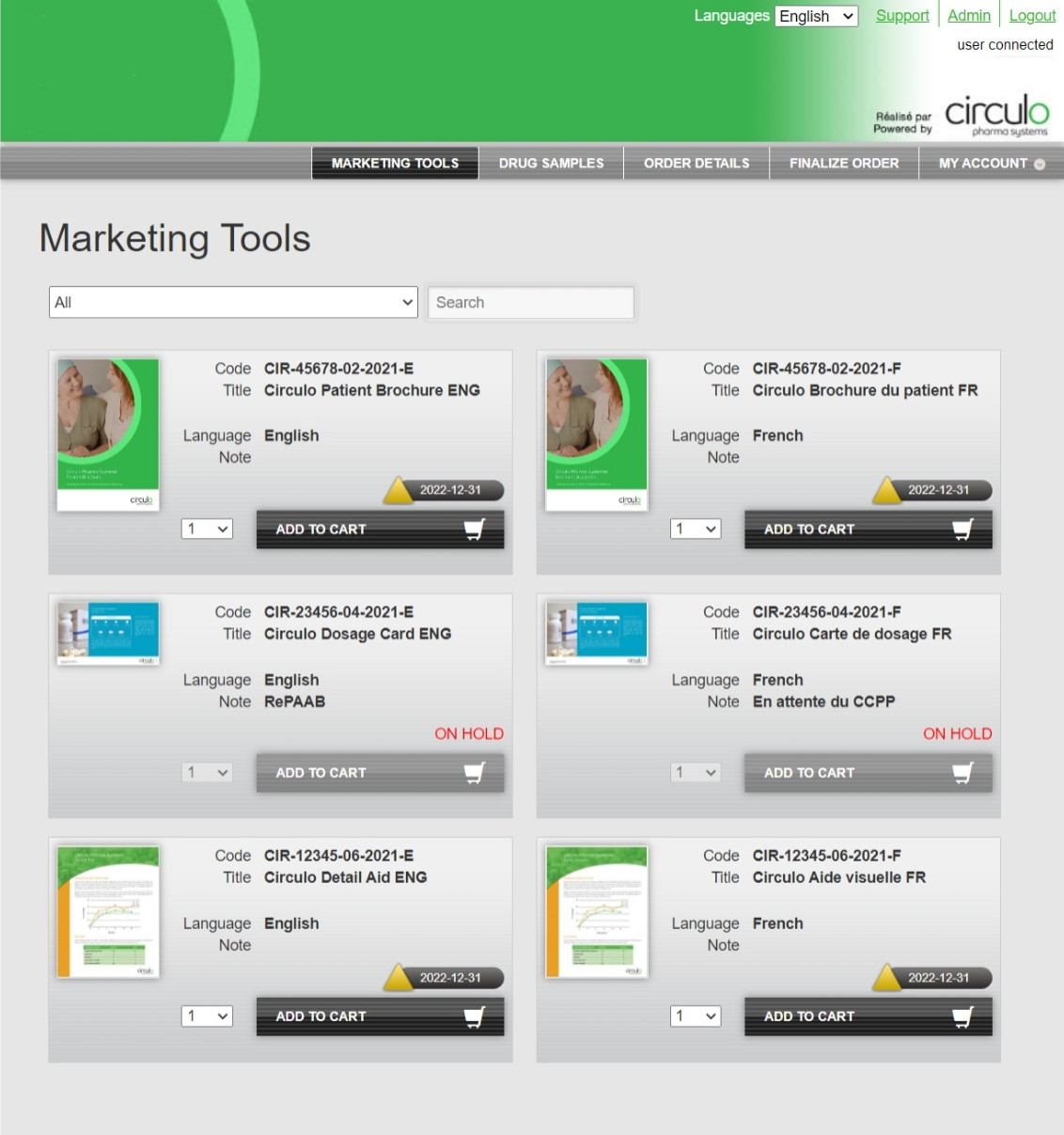
Our secure web-based portal enables your sales team to order marketing material and product samples in one central location, saving time and reducing workload. The system also aids in compliance, featuring automatic product and material recalls, real-time scheduling of paperless field audits, and inventory tracking.
- 24/7 access to order entry & tracking
- Validated to the latest standards
- Multi-level user access
- Easy-to-use user interface
- Integrates with existing order and distribution tracking platforms
- CRM integration
- Customizable reporting of inventory and rep activity
Everything Circulo has to offer
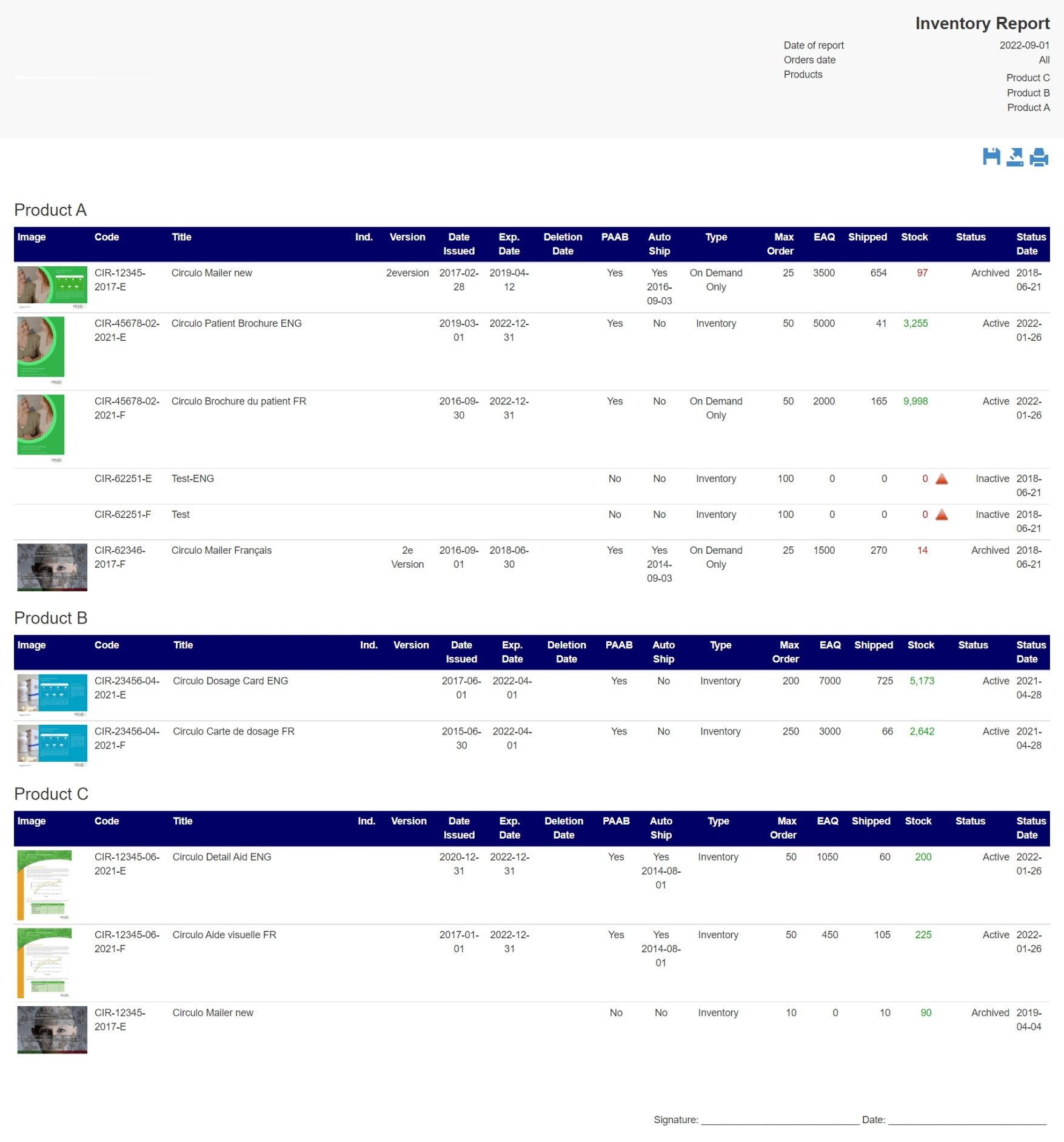
Inventory Control & Management
Reduce operating costs by optimizing warehouse operations and reducing inventory holding costs. Get high-volume, multi-channel eCommerce management, keep your inventory up-to-date, and minimize cost.
Streamlined Approval Management
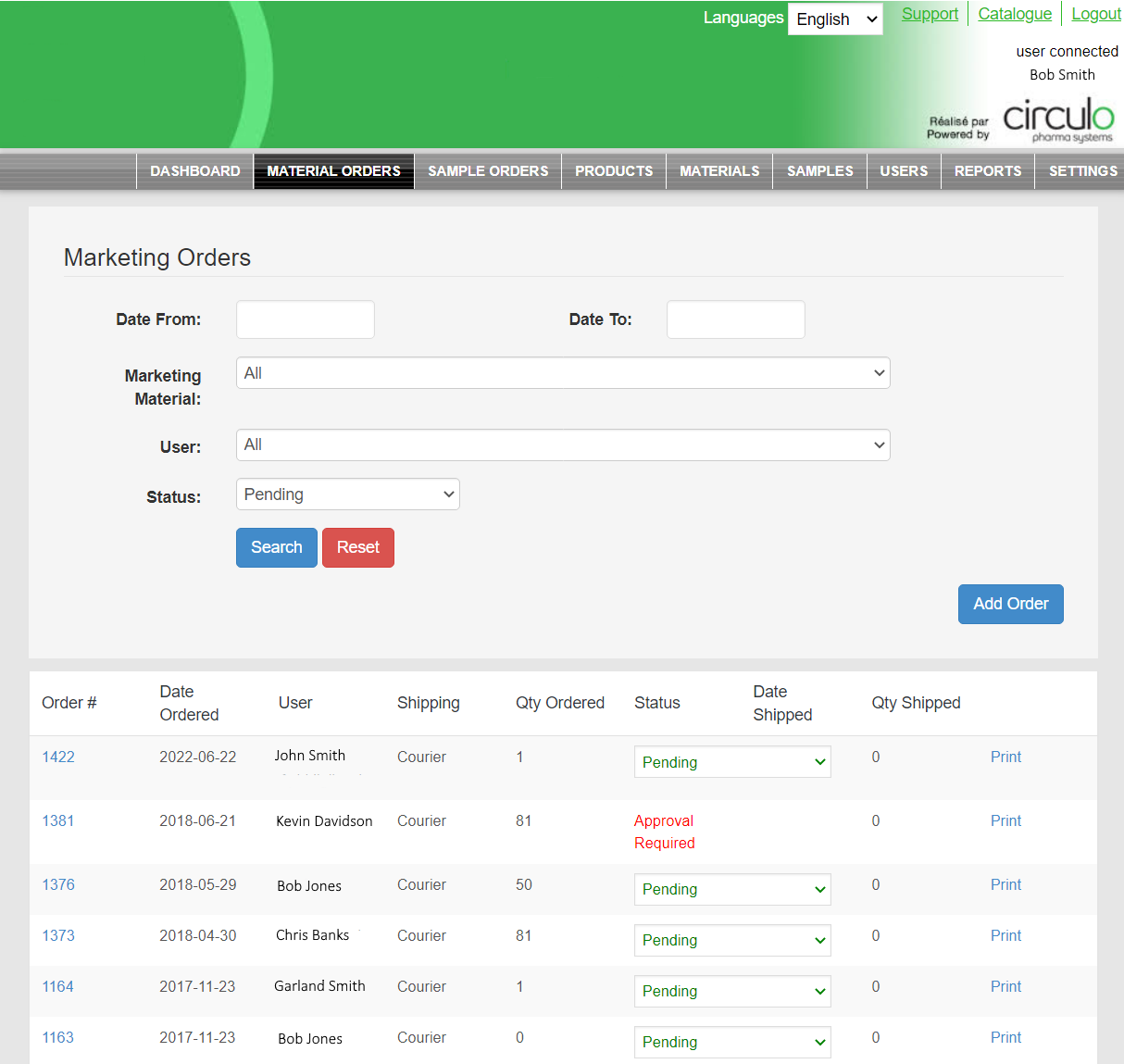
With the Circulo portal, you can configure policies to require approval for access to ordering marketing material and samples. Set materials to automatically expire and send alerts to anyone using them.
Distribution Management
Systemize your inventory and distribution across all sales channels. Any change you make in your final stock is reflected instantly in all your sales channels.
Workflow Management
Automate and track business processes with configurable workflows that provide assignments, routing, approval email notifications, escalation, and tracking of work items.
Oversight Management and Analytics
Quickly analyze and compare data across multiple dimensions to gain actionable insights! Circulo’s analytics provide a simple and accessible toolset, enabling you to quickly give your business more time and focus on what matters – your customers
Security and Compliance Features
A logically designed software development process tested to perform as expected. The platform is also developed for cloud-based deployment and the ability to perform ongoing maintenance and upgrades.
The Circulo platform is developed and maintained to meet U.S. National Institute of Standards and Technology (NIST) guidelines for mitigating organizational cybersecurity risks.
The Circulo team meets national privacy laws in how the company operates and how the platform is developed, including HITECH/GLBA, E.U. GDPR, Canada PIPEDA, Mexico and Latin American DPAs, and PDPAs, Australia PA, Asian PIPAs, and PDPAs.
Comply with FDA guidelines under which electronic records and signatures are trustworthy, reliable, and equivalent to paper records.
Both business and platform-level capabilities have been evaluated by a 3rd party to ensure the systems and operations remain operational in case of disaster. This includes support for multiregion deployments for failover, plans for application operations with reduced functionality or degraded performance during an outage, and consistent backups for applications and data.
All branded marketing tools utilize expiry dates and can automatically send recall notices when dated material must be recovered for disposal.
All integrated hardware and software components support the isolated processing, tracking, and storage of all information. There is no co-mingling of customer data or sharing of platform resources. Each environment is isolated from the other.
Each environment has a private sub-domain registry that allocates domain names in a subset of the primary Domain Name System under a domain registered with an ICANN-accredited or ccTLD registry.

SDLC Validation
A logically designed software development process tested to perform as expected. The platform is also developed for cloud-based deployment and the ability to perform ongoing maintenance and upgrades.
Modeled to meet NIST Cybersecurity Framework
The Circulo platform is developed and maintained to meet U.S. National Institute of Standards and Technology (NIST) guidelines for mitigating organizational cybersecurity risks.

Meets national and international privacy laws
The Circulo team meets national privacy laws in how the company operates and how the platform is developed, including HITECH/GLBA, E.U. GDPR, Canada PIPEDA, Mexico and Latin American DPAs, and PDPAs, Australia PA, Asian PIPAs, and PDPAs.
21 CFR (Part 11) compliant data capture
Comply with FDA guidelines under which electronic records and signatures are trustworthy, reliable, and equivalent to paper records.

Enhanced Business Continuity Disaster Recovery (BCDR)
Both business and platform-level capabilities have been evaluated by a 3rd party to ensure the systems and operations remain operational in case of disaster. This includes support for multiregion deployments for failover, plans for application operations with reduced functionality or degraded performance during an outage, and consistent backups for applications and data.
PAAB Compliant (Pharmaceutical Advertising Advisory Board)
All branded marketing tools utilize expiry dates and can automatically send recall notices when dated material must be recovered for disposal.

Dedicated data environment and private sub-domain registry
All integrated hardware and software components support the isolated processing, tracking, and storage of all information. There is no co-mingling of customer data or sharing of platform resources. Each environment is isolated from the other.
Each environment has a private sub-domain registry that allocates domain names in a subset of the primary Domain Name System under a domain registered with an ICANN-accredited or ccTLD registry.
